Key Compliance Requirements Under MD 4 & MD 6 for Medical Device Manufacturers
Form MD 4 and MD 6 are critical provisions of the Medical Device Rules, 2017.
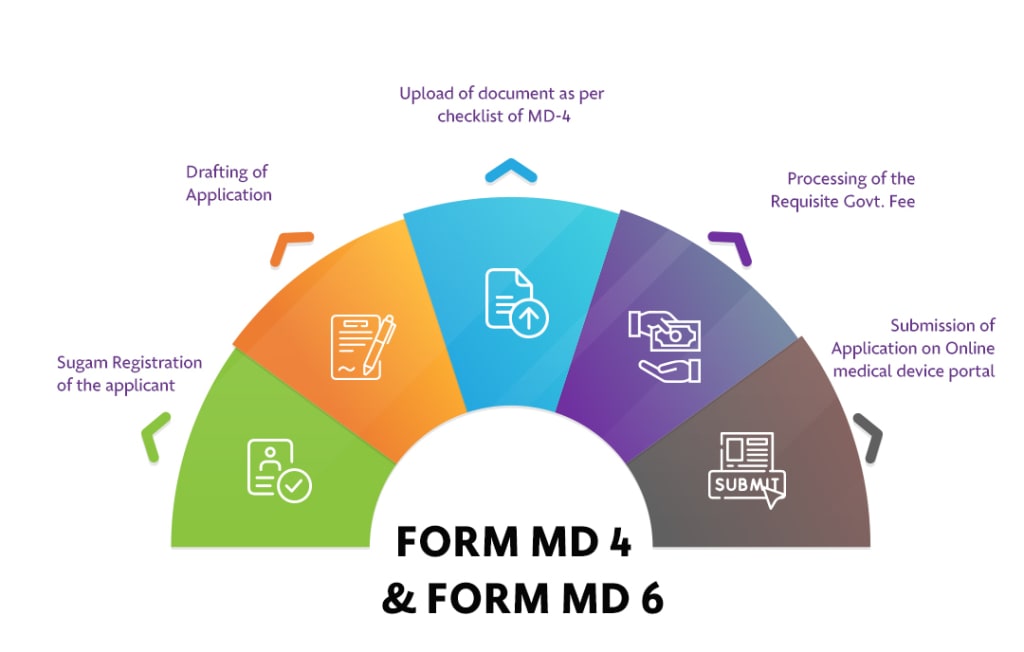
Summary
- Form MD 4 is used for the licensing application, whereas Form MD 6 grants the manufacturing license required to produce medical devices in India.
- Application Process: Submit Form MD-7, provide supporting documents, document verification, pay fees, and approval or issuance of registration certificate.
- Manufacturers are required to submit a registration application through CDSCO’s online portal.
- Compliance with Form MD 4 and Form MD 6 is mandatory for manufacturers to obtain market authorization and manufacturing licenses.
Short Description
To ensure the safety, quality, and efficacy of medical devices produced in the Indian ecosystem, the Central Drugs Standard Control Organization (CDSCO) introduced the Medical Device Rules (MDR), 2017 under the Drugs and Cosmetics Act.
Manufacturers are required to adhere to Forms MD 4 and MD 6 under the MDR, 2017. This blog outlines the compliance requirements for obtaining manufacturing licenses, offering manufacturers a seamless route for navigating India’s regulatory channels.
Form MD 4: Registration of Medical Devices for Manufacturers and Importers
Form MD 4 pertains to the application procedure for the registration of medical devices in India. Manufactures aiming to obtain a manufacturing license must submit the application through CDSCO’s online portal.
Due to stringent quality compliance and safety and efficacy of medical devices produced, manufacturers are required to engage in comprehensive documentation process.
Mandatory Documents for MD 4 Registration
Here are the necessary documents required for MD 4 registration:
Device Master File (DMF): Document containing information about the device’s design, materials used, manufacturing process, intended use, materials, and performance data.
Plant Master File (PMF): Document containing details about the manufacturing site, including everything from infrastructure to quality control procedures.
Certificate of Free Sale: In the case of imported medical devices, a certificate from the manufacturing country indicating the legal marketing of that device in the origin country.
Risk Classification Document: Classification of the medical device (Class A, B, C, or D), according to its risk level.
Unique Device Identification (UDI): A unique identifying number allocated to each medical device to ensure its traceability.
Steps for MD 4 Registration
Application Submission: Submit the application for manufacturing license, along with necessary documents, through CDSCO’s online portal.
Document Verification: The CDSCO or the Authorised Body verifies the documents submitted to ensure strict compliance with the regulatory standards.
Approval or Rejection of the Application: Once approved, the CDSCO issues a registration certificate granting the legal marketing of medical devices in India.
Form MD 6: Grant of Manufacturing License for Medical Devices in India
Form MD 6 grants the license to manufacture medical devices for sale and distribution to medical device manufacturers.
In the licensing process, the authorities ensure the manufacturing site is compliant with Good Manufacturing Practices (GMP) and the medical devices meet strict safety and quality standards.
Key Compliance Requirements Under the Form MD 6
Evaluation of Manufacturing Facility by Regulatory Authorities: Prior to administering the manufacturing license, the CDSCO conducts a comprehensive inspection of the manufacturing site to confirm adherence to Good Manufacturing Practices (GMP) regulations.
Appointment of an Authorised Signatory: Manufacturers are required to designate a qualified individual to oversee compliance with regulatory requirements.
Provision of Manufacturing Site Information: Manufacturers must submit the manufacturing facility’s layout, production methods, quality control procedures, and qualifications of personnel.
Compliance With Quality Management Systems (QMS): Manufacturers are required to implement a QMS in accordance with ISO 13485, which specifies the criteria for a complete QMS tailored to medical devices.
Regular Audits and Inspections: After receiving the manufacturing license, manufacturers are obligated to periodic audits and inspections to ensure continued compliance with regulatory requirements.
Validity of Form MD-6 Manufacturing License
After the application procedure, it takes about 4 to 5 months to receive the Form MD-6 manufacturing license from the State Licensing Authority. Once the license (Form MD-6) is issued, it remains valid till perpetuity.
Best Practices for Ensuring Compliance
Manufacturers can follow the following best practices to enhance their adherence to MD 4 and MD 6:
Create a Regulatory Affairs Team: A specialized team can oversee documentation, track regulatory updates, and communicate with regulatory bodies.
Adopt a Quality Management System (QMS): Compliance with ISO 13485 guarantees that manufacturers align with international quality benchmarks.
Perform Regular Internal Audits: Frequent audits assist in recognizing and resolving compliance issues before regulatory inspections occur.
Keep Detailed Records: Precise and current records assist in expediting regulatory approvals and ease the audit process.
Conclusion
Adherence to MD 4 and MD 6 is crucial for medical device manufacturers to guarantee that their products meet safety, quality, and performance criteria.
The Indian regulatory framework established by the Medical Device Rules, 2017, is designed to protect public health by ensuring that only compliant devices are available in the marketplace.
Although navigating the regulatory environment can be complex, embracing best practices and remaining aware of changing requirements can assist manufacturers in achieving smooth compliance.
As the Indian medical device market continues to expand, following regulatory obligations will continue to be a vital element for success. Manufacturers should perceive compliance not as a barrier, but as a chance to improve product quality, foster market confidence, and contribute to a more secure healthcare environment.
If you are seeking assistance in the manufacturing license process, CliniExperts can help you with its expertise in license procurement while adhering to regulatory requirements.
With a successful proven record of guiding manufacturers through the licensing process, streamlining timely approvals, and navigating through complex regulatory landscape, CliniExperts can help you stay on top of the regulatory compliances.





Comments (1)
I love all the information you post 🏆⭐️⭐️⭐️⭐️⭐️⭐️⭐️