Europe Non-Invasive Prenatal Testing Market Size & Forecast 2025–2033: A Safer Path to Smarter Prenatal Care
Rising maternal age, supportive regulations, and precision genetics push Europe’s NIPT market toward US$ 1.99 billion by 2033
Europe’s NIPT Moment: From Niche Test to Mainstream Screening
Europe’s healthcare systems are in the middle of a quiet but powerful shift in how pregnancies are screened for chromosomal conditions—and non-invasive prenatal testing (NIPT) sits right at the center of it. Once considered a premium, optional test, NIPT is increasingly becoming part of routine prenatal pathways across several European countries, especially for high-risk pregnancies. The reason is simple: it combines safety, accuracy, and early insight in a single blood draw from the mother.
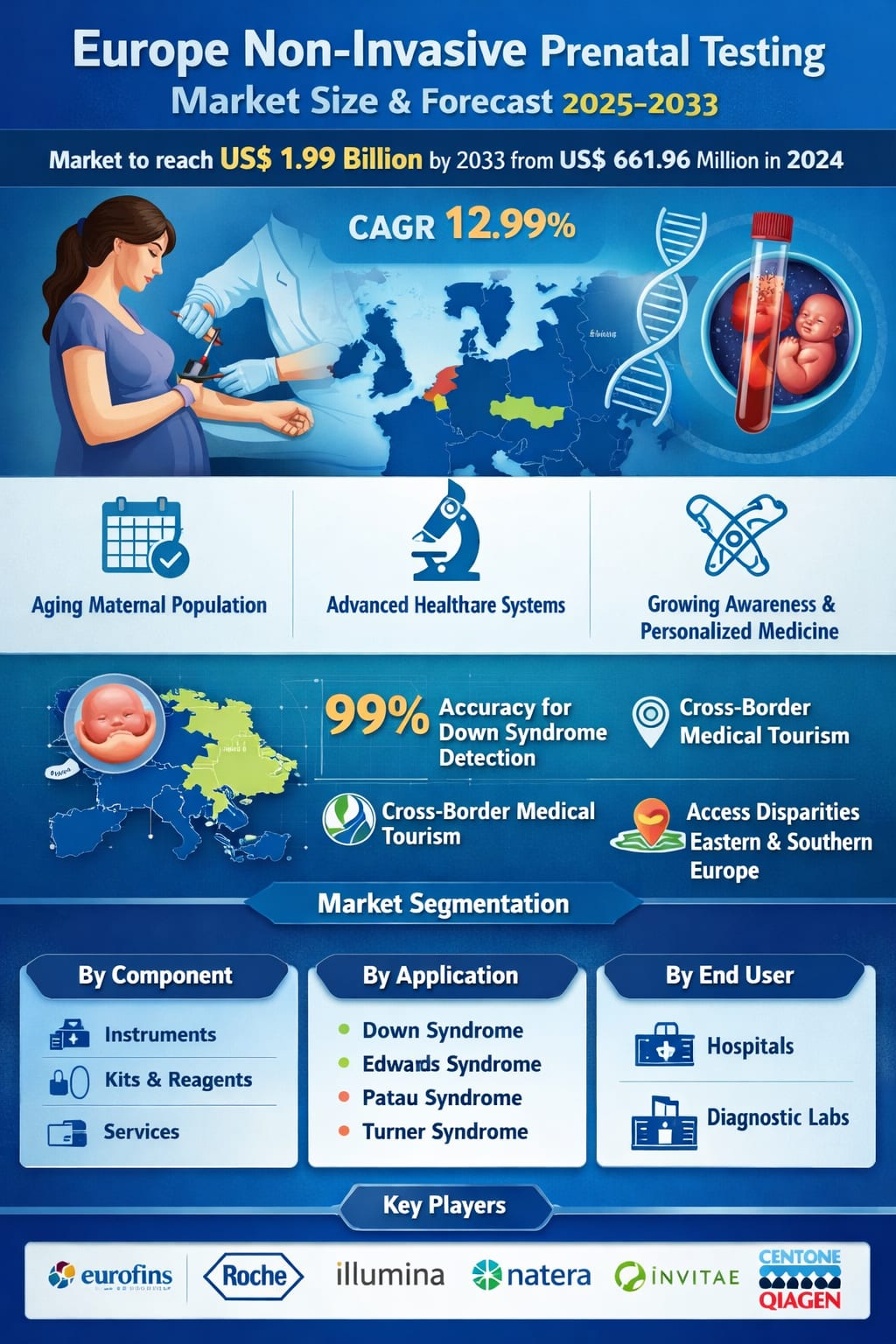
According to Renub Research, the Europe Non-Invasive Prenatal Testing Market is expected to grow from US$ 661.96 million in 2024 to US$ 1,987.07 million by 2033, expanding at a CAGR of 12.99% from 2025 to 2033. That’s not just steady growth—it’s a clear signal that European healthcare is embracing genomic screening as a core pillar of modern prenatal care.
At its heart, NIPT analyzes fragments of fetal DNA circulating in maternal blood to screen for the most common chromosomal abnormalities, including Down syndrome (Trisomy 21), Edwards syndrome (Trisomy 18), Patau syndrome (Trisomy 13), and conditions such as Turner syndrome. With reported detection rates exceeding 99% for Down syndrome in many clinical settings, the test has earned trust from clinicians and expectant parents alike—especially because it avoids the miscarriage risks associated with invasive procedures like amniocentesis or chorionic villus sampling.
Why Europe Is Leaning Into NIPT
Several structural and social trends are converging to push NIPT into the mainstream across Europe.
1) Rising maternal age.
Across the continent, women are increasingly choosing to have children later in life due to career priorities, economic considerations, and lifestyle changes. Eurostat data has long shown a steady rise in the average age of first-time mothers, and with age comes a higher statistical risk of chromosomal abnormalities. This demographic shift alone creates a strong, lasting demand for accurate early screening—precisely where NIPT shines.
2) Strong healthcare infrastructure.
Countries such as Germany, France, the United Kingdom, and the Netherlands have well-developed public healthcare systems capable of integrating advanced diagnostics into standard care pathways. In many of these markets, NIPT is already recommended—especially for high-risk pregnancies—either through public reimbursement schemes or structured referral programs.
3) The push toward personalized medicine.
Europe’s healthcare strategy is increasingly focused on predictive, preventive, and personalized care. NIPT fits neatly into this philosophy by offering patient-specific genetic risk information early in pregnancy, helping clinicians and families make informed decisions without exposing the fetus to unnecessary risk.
4) Regulatory and clinical guideline support.
Across Europe, regulators and professional medical bodies have built clearer frameworks around genetic testing, laboratory standards, data privacy, and ethical use. This has increased clinician confidence and normalized NIPT as a reliable screening tool rather than a fringe or luxury option.
Market Headwinds: Where the Challenges Still Lie
Despite its strong growth trajectory, the European NIPT market is not without friction.
Uneven access across countries remains one of the biggest issues. While Western and Northern European nations have made significant progress in integrating NIPT into public healthcare, parts of Eastern and Southern Europe still struggle with limited funding, inconsistent reimbursement policies, and weaker diagnostic infrastructure. The result is a patchwork of access levels that creates real inequalities in prenatal care.
Public misunderstanding and misinformation also present challenges. Many patients still confuse NIPT with a diagnostic test rather than a highly accurate screening tool. Without proper counseling, positive or inconclusive results can cause unnecessary anxiety. Expanding access to genetic counseling and improving patient education will be crucial for the market’s long-term, sustainable growth.
Cross-Border Healthcare: A Quiet Growth Engine
One uniquely European driver is cross-border healthcare and medical tourism. Thanks to freedom of movement within the EU, expectant parents increasingly travel to neighboring countries to access faster, more affordable, or more advanced NIPT services. Private clinics in countries like Germany, Belgium, and the Netherlands actively attract international patients, boosting test volumes and encouraging competition on quality, turnaround time, and pricing. This cross-border dynamic not only expands the market but also helps standardize quality benchmarks across the region.
Market Segmentation: Where the Growth Is Concentrated
Renub Research segments the Europe NIPT market across component, application, and end user, offering a clear picture of where value is being created.
By Component:
Instruments
Kits and Reagents
Services
While instruments form the technological backbone of NIPT, kits, reagents, and testing services account for a significant share of recurring revenue, driven by the growing number of tests performed annually.
By Application:
Down Syndrome (Trisomy 21)
Edwards Syndrome (Trisomy 18)
Patau Syndrome (Trisomy 13)
Turner Syndrome
Other Applications
Screening for Down syndrome remains the dominant application segment, reflecting both its higher prevalence and the strong clinical emphasis on early detection.
By End User:
Hospitals
Diagnostic Labs
While hospitals play a key role in patient referral and integrated prenatal care, diagnostic laboratories are the operational engine of the market, processing high volumes of tests and driving innovation in testing platforms and workflows.
Country Spotlight: A Closer Look at Key European Markets
France
France’s NIPT market is growing steadily, supported by increasing awareness of prenatal screening and the healthcare system’s gradual integration of NIPT into routine care for high-risk pregnancies. The main challenges remain equitable access—especially in rural areas—and the availability of trained genetic counselors to help patients interpret results and make informed choices.
Germany
Germany stands out as one of Europe’s most advanced NIPT markets, backed by strong healthcare infrastructure and rapid adoption of genetic testing technologies. NIPT is widely used for high-risk pregnancies, and awareness among both clinicians and patients is high. Future growth will depend on expanding counseling services and ensuring access across all socioeconomic groups.
United Kingdom
In the UK, NIPT has become a crucial part of prenatal screening, particularly for women identified as higher risk through initial screening tests. The test is available through both public and private channels, although challenges remain around regional access disparities, cost considerations, and counseling capacity. Continued policy support and investment in healthcare infrastructure are expected to strengthen adoption further.
Other important markets include Italy, Spain, Belgium, the Netherlands, and Turkey, each contributing to regional growth at different speeds depending on healthcare policy, reimbursement structures, and public awareness.
Competitive Landscape: Science, Scale, and Strategy
The European NIPT market is shaped by a mix of global life science leaders and specialized genetic testing companies. Key players covered in the market include:
Eurofins Scientific
F. Hoffmann-La Roche Ltd
Invitae Corporation
Illumina Inc.
Natera Inc.
Centogene NV
Qiagen
These companies compete across multiple fronts: test accuracy, turnaround time, cost efficiency, laboratory automation, and data interpretation tools. Strategic partnerships with hospitals, diagnostic networks, and national healthcare systems are becoming just as important as pure technological innovation. Recent years have also seen a focus on expanding test menus, improving bioinformatics pipelines, and scaling laboratory capacity to meet rising demand.
The Bigger Picture: NIPT and Europe’s Healthcare Future
What makes the NIPT story in Europe particularly compelling is how closely it aligns with broader healthcare priorities. Europe is aging, healthcare budgets are under pressure, and policymakers are increasingly focused on early detection, prevention, and cost-effective care pathways. NIPT supports all three by reducing the need for invasive procedures, lowering complication risks, and enabling better-informed clinical decisions early in pregnancy.
At the same time, ethical oversight and patient rights remain central to the European approach. Data protection, informed consent, and responsible use of genetic information are not side notes—they are core pillars of how the market is being shaped. This balanced approach is one reason Europe’s NIPT market is growing not just fast, but also sustainably.
Market Outlook: From Growth to Normalization
With a projected market size of US$ 1.99 billion by 2033, Europe’s NIPT sector is clearly moving from a growth phase into a phase of normalization and integration. In the coming years, the conversation will likely shift from “Should we use NIPT?” to “How can we make NIPT accessible, affordable, and well-supported for everyone who needs it?”
Expect continued investment in:
Laboratory automation and high-throughput testing
AI-driven data interpretation and reporting
Training and expansion of genetic counseling services
Cross-border collaborations and standardized clinical pathways
As these pieces fall into place, NIPT is poised to become not just a premium option, but a routine, trusted part of prenatal care across much of Europe.
Final Thoughts
The Europe Non-Invasive Prenatal Testing market is a powerful example of how technology, demographics, and healthcare policy can align to transform clinical practice. Driven by rising maternal age, strong healthcare systems, supportive regulations, and the shift toward personalized medicine, the market’s growth from US$ 661.96 million in 2024 to US$ 1,987.07 million by 2033 reflects more than just commercial opportunity—it reflects a change in how Europe approaches prenatal care itself.
Challenges remain, particularly around equitable access and patient education, but the direction of travel is clear. NIPT is no longer a niche innovation. It is fast becoming a cornerstone of modern, patient-centered prenatal screening in Europe—and the next decade will likely cement its role as a standard of care rather than a specialized add-on.





Comments
There are no comments for this story
Be the first to respond and start the conversation.