Saudi Arabia Generic Drugs Market Size & Forecast 2025–2033
Affordable Medicines Powering the Kingdom’s Healthcare Transformation
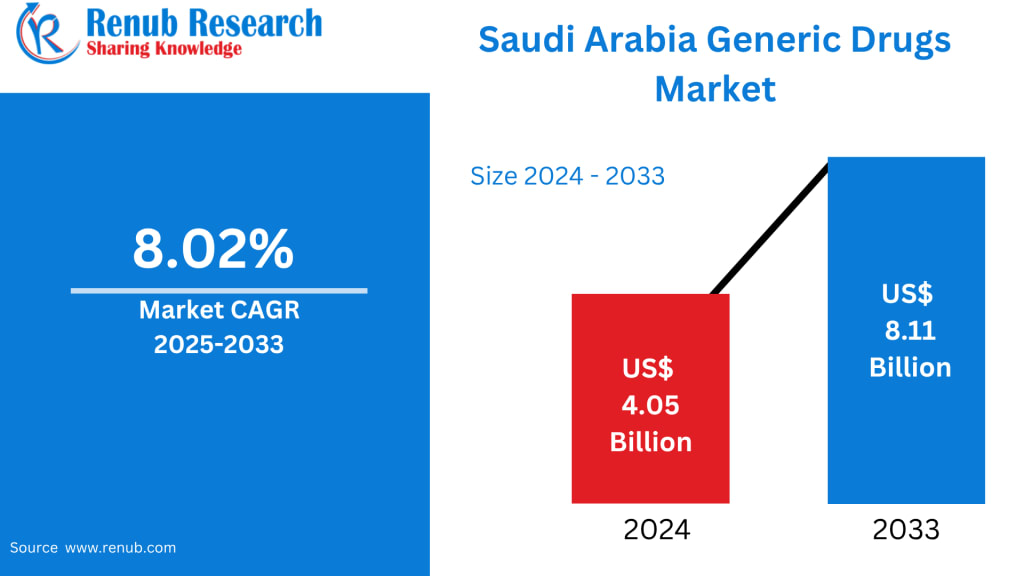
Market Snapshot (Renub Research)
The Saudi Arabia Generic Drugs Market is projected to expand from US$ 4.05 billion in 2024 to US$ 8.11 billion by 2033, registering a strong CAGR of 8.02% during 2025–2033. This growth trajectory is driven by rising healthcare demand, government-led cost-containment policies, increasing chronic disease prevalence, and sustained support for local pharmaceutical manufacturing.
Saudi Arabia Generic Drugs Market Overview
Generic drugs are bioequivalent versions of branded medicines, offering the same active ingredients, dosage strength, safety, quality, and therapeutic efficacy, but at significantly lower prices. These medicines undergo stringent bioequivalence testing and regulatory approval before entering the market, ensuring patient confidence and clinical effectiveness.
In Saudi Arabia, generic drugs are becoming a cornerstone of the healthcare system. Policymakers and healthcare providers increasingly recognize generics as an effective solution to reduce pharmaceutical expenditure, expand patient access, and ensure long-term system sustainability. Regulatory facilitation by the Saudi Food and Drug Authority (SFDA) has accelerated generic approvals, encouraging both domestic and international manufacturers to strengthen their presence in the Kingdom.
Combined with population growth, rising healthcare awareness, and the government’s focus on preventive care, generics are steadily gaining acceptance among physicians, pharmacists, insurers, and patients alike.
Key Growth Drivers
Government Support and Cost-Containment Initiatives
Saudi Arabia’s healthcare policy strongly favors affordable treatment options. The government actively promotes generic drug adoption across public hospitals and insurance programs to curb escalating healthcare costs. Streamlined approval pathways, pricing regulations, and incentives for domestic production have significantly boosted market momentum.
A notable regulatory step was the SFDA’s draft initiative titled “Procedure to Deal with Patents When Registering Generic Products”, released for public consultation in 2022. This framework clarified patent-related pathways for generics, reducing uncertainty for manufacturers and enhancing market confidence. Such policy clarity continues to attract foreign pharmaceutical investments into the Kingdom.
Rising Burden of Chronic Diseases
Saudi Arabia faces one of the highest chronic disease burdens in the Middle East. Conditions such as diabetes mellitus, hypertension, cardiovascular disorders, obesity, and respiratory diseases require lifelong medication, significantly increasing treatment costs.
Generic drugs offer a sustainable solution for managing chronic illnesses without compromising quality. As physicians and patients seek cost-effective long-term therapies, generics are becoming the default choice. The growing elderly population and sedentary lifestyles further reinforce consistent demand across therapeutic categories.
Expansion of Local Manufacturing Capacity
Under Saudi Vision 2030, the Kingdom is aggressively expanding its domestic pharmaceutical manufacturing base. Investments in advanced production facilities, technology transfer agreements, and public–private partnerships are strengthening local capabilities.
In January 2024, Saudi Arabia announced a national biotechnology strategy aimed at positioning the country as a global biotech hub within 16 years. This initiative supports not only innovative drugs but also advanced generics and biosimilars, reducing import dependence and improving supply security.
Market Challenges
Public Perception and Brand Loyalty
Despite clinical equivalence, branded medicines still enjoy strong trust among segments of the population. Cultural perceptions, physician prescribing habits, and historical reliance on multinational brands can slow generic adoption. Overcoming this challenge requires continued education campaigns, physician engagement, and transparent quality assurance.
Regulatory and Quality Compliance Complexity
While approvals have become faster, generic manufacturers must still meet rigorous requirements for bioequivalence, Good Manufacturing Practices (GMP), and post-market surveillance. For smaller or new entrants, these compliance costs can be substantial. Maintaining consistent quality and preventing counterfeit medicines also remain ongoing operational challenges.
Market Segmentation Insights
Saudi Arabia Simple Generic Drugs Market
Simple generics—medicines with uncomplicated formulations—dominate the Saudi market. Widely used for pain management, infections, hypertension, and common ailments, they are easy to manufacture and face fewer regulatory complexities. Government price controls and widespread use in public hospitals ensure high volumes, making this segment an attractive entry point for manufacturers.
Saudi Arabia Specialty Generic Drugs Market
Specialty generics address complex and high-cost therapies, including oncology, autoimmune diseases, and rare disorders. As Saudi Arabia seeks to reduce reliance on expensive imported biologics, investment in specialty generics is rising. Though development costs are higher, these products offer substantial savings for healthcare systems and insurers, driving long-term growth.
Saudi Arabia Oral Generic Drugs Market
Oral dosage forms—tablets, capsules, and syrups—remain the most preferred due to ease of administration and patient compliance. Strong manufacturing infrastructure and widespread outpatient usage ensure oral generics maintain the largest market share, particularly for chronic disease management.
Saudi Arabia Respiratory Generic Drugs Market
Environmental factors such as dust exposure and smoking contribute to high asthma and COPD prevalence. Demand for affordable inhalers, MDIs, DPIs, and nebulized formulations is steadily increasing. Although device complexity poses regulatory challenges, supportive reimbursement policies and public health initiatives continue to expand access.
Saudi Arabia Oncology Generic Drugs Market
Cancer treatment costs place heavy pressure on healthcare budgets. Oncology generics, including biosimilars, are gaining traction as hospitals integrate them into treatment protocols. Government funding, insurance coverage, and early diagnosis programs support adoption, enabling equitable cancer care across income groups.
Saudi Arabia Online Generic Drugs Market
Digital healthcare transformation has fueled rapid growth in online pharmacies and e-prescription platforms. Accelerated by COVID-19, consumers now benefit from convenience, price transparency, and home delivery. High smartphone penetration and tech-savvy urban populations in Riyadh and Jeddah are key demand drivers.
Regional Market Analysis
Western Saudi Arabia
Cities such as Jeddah, Mecca, and Medina form a major consumption hub due to dense populations, advanced healthcare infrastructure, and pilgrimage-related healthcare demand. Public and private hospitals actively support generic usage, aligning with national affordability goals.
Eastern Saudi Arabia
The Eastern region, including Dammam and Khobar, benefits from strong logistics infrastructure and expanding healthcare facilities. Rising insurance coverage and chronic disease prevalence are accelerating generic drug adoption across both public and private sectors.
Market Segmentation Summary
By Type
Simple Generics
Specialty Generics
Biosimilars
By Route of Administration
Oral
Injections
By Therapeutic Area
Infectious Diseases
Respiratory
Musculoskeletal
Oncology
CNS
Cardiovascular
Others
By Distribution Channel
Online Pharmacies
Retail Pharmacies
Hospital Pharmacies
By Region
Northern & Central
Western
Eastern
Southern
Competitive Landscape & Key Players
The Saudi generic drugs market is moderately consolidated, with global and regional leaders competing on quality, pricing, and local partnerships. Each company is analyzed across five viewpoints: Overview, Key Person, Recent Developments, SWOT Analysis, and Revenue Analysis.
Major Companies Operating in Saudi Arabia
Teva Pharmaceutical Industries Ltd.
Viatris Inc.
Sandoz Group AG
Sun Pharmaceutical Industries Ltd.
Cipla Ltd.
Aurobindo Pharma Ltd.
Lupin Ltd.
Hikma Pharmaceuticals PLC
STADA Arzneimittel AG
Dr. Reddy’s Laboratories Ltd.
Final Thoughts
The Saudi Arabia Generic Drugs Market is entering a decisive growth phase. With strong government backing, rising chronic disease prevalence, expanding local manufacturing, and accelerating digital healthcare adoption, generics are becoming indispensable to the Kingdom’s healthcare future.
As Vision 2030 continues to reshape the pharmaceutical ecosystem, generic drugs will play a critical role in improving access, controlling costs, and ensuring long-term sustainability. For manufacturers, investors, and healthcare stakeholders, Saudi Arabia represents one of the most promising generic drug markets in the Middle East through 2033.
About the Creator
Marthan Sir
Educator with 30+ years of teaching experience | Passionate about sharing knowledge, life lessons & insights | Writing to inspire, inform, and empower readers.






Comments
There are no comments for this story
Be the first to respond and start the conversation.