United States Alzheimer’s Drugs Market Size & Forecast 2025–2033
Market Transformation Driven by Disease-Modifying Therapies and an Aging Nation
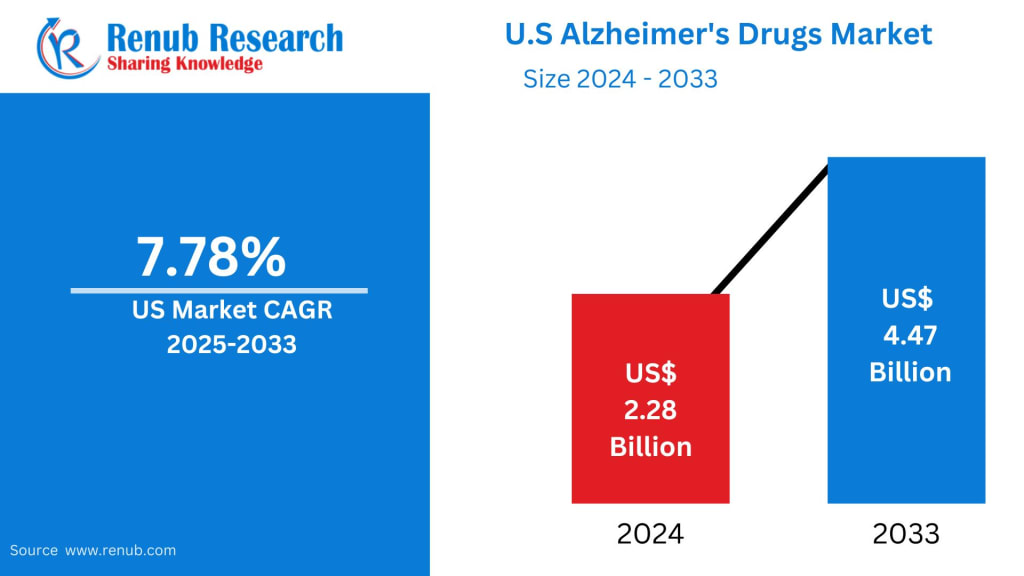
United States Alzheimer’s Drugs Market Outlook
The United States Alzheimer’s Drugs Market is entering a pivotal transformation phase, supported by scientific breakthroughs, demographic shifts, and heightened public health prioritization. According to Renub Research estimates, the market is projected to expand from US$ 2.28 billion in 2024 to US$ 4.47 billion by 2033, registering a CAGR of 7.78% during 2025–2033.
This robust growth is fueled by the rising incidence of Alzheimer’s disease, an expanding elderly population, and the emergence of disease-modifying therapies that aim to slow disease progression rather than merely manage symptoms. Recent FDA approvals of monoclonal antibody therapies, combined with significant investments from government agencies and private biotech firms, are redefining the treatment paradigm in the U.S.
United States Alzheimer’s Drugs Market Overview
Alzheimer’s disease is a progressive neurodegenerative disorder characterized by memory loss, cognitive decline, and behavioral changes. It primarily affects individuals aged 65 and above, though early-onset cases are also reported. The condition poses a significant social, economic, and emotional burden on patients, caregivers, and the healthcare system.
Alzheimer’s drugs broadly fall into two categories:
Symptomatic treatments, including cholinesterase inhibitors (donepezil, rivastigmine, galantamine) and NMDA receptor antagonists (memantine), which help manage memory loss and cognitive symptoms.
Disease-modifying therapies, such as monoclonal antibodies targeting beta-amyloid plaques (e.g., lecanemab), designed to slow cognitive decline.
In the U.S., more than 6 million people currently live with Alzheimer’s disease, and this number is expected to rise sharply as life expectancy increases. Growing awareness, early diagnosis initiatives, and improved access to neurological care are accelerating the adoption of pharmaceutical treatments nationwide.
Key Growth Drivers in the United States Alzheimer’s Drugs Market
Aging Population and Rising Disease Prevalence
The United States is witnessing a rapid demographic shift. The population aged 65 and above continues to grow, significantly increasing the number of individuals at risk of Alzheimer’s disease. According to estimates by the Alzheimer’s Association, the number of Americans living with Alzheimer’s could nearly double by 2050.
This demographic trend is driving sustained demand for both early-stage symptomatic therapies and advanced disease-modifying drugs. As diagnosis rates improve, long-term treatment regimens are becoming more common, strengthening market expansion.
Breakthrough Drug Development and FDA Approvals
The approval of amyloid-targeting monoclonal antibodies has marked a historic shift in Alzheimer’s treatment. Drugs such as lecanemab and aducanumab are redefining clinical expectations by attempting to alter disease progression rather than offering temporary symptom relief.
Accelerated FDA pathways and adaptive clinical trial designs have encouraged pharmaceutical companies to expand R&D pipelines aggressively. In March 2025, Modulo Bio received a US$ 4.8 million grant from the Alzheimer’s Drug Discovery Foundation (ADDF) to advance neurodegenerative research, highlighting strong funding momentum in this space.
Government Support and Research Funding
Alzheimer’s disease is recognized as a national public health priority in the U.S. Federal initiatives such as the National Alzheimer’s Project Act (NAPA) and increased funding for the National Institute on Aging (NIA) have strengthened research ecosystems.
Public–private partnerships involving academic institutions, biotech startups, and established pharmaceutical firms are shortening drug development timelines and accelerating commercialization. These initiatives are improving early diagnosis capabilities and expanding patient access to innovative therapies.
Challenges in the United States Alzheimer’s Drugs Market
High Cost and Affordability Constraints
Despite scientific progress, the high cost of newly approved Alzheimer’s therapies remains a significant barrier. Disease-modifying monoclonal antibodies can cost tens of thousands of dollars annually, excluding diagnostic imaging and monitoring expenses such as PET scans and MRIs.
Limited insurance reimbursement and strict eligibility criteria restrict access, particularly for middle- and lower-income patients. Without broader Medicare and private insurance coverage, adoption of advanced therapies may remain constrained.
Late Diagnosis and Diagnostic Infrastructure Gaps
Early diagnosis is critical for effective Alzheimer’s treatment, especially for disease-modifying drugs. However, many patients in the U.S. are diagnosed at moderate or advanced stages due to limited access to specialists, high diagnostic costs, and social stigma around cognitive testing.
Rural and underserved regions face a shortage of neurologists and memory care centers, delaying treatment initiation and reducing overall drug utilization.
United States Alzheimer’s Drugs Market by Drug Class
Cholinesterase Inhibitors Market
Cholinesterase inhibitors remain the backbone of Alzheimer’s treatment in the U.S., particularly for mild to moderate cases. Drugs such as donepezil, galantamine, and rivastigmine enhance acetylcholine levels, improving communication between nerve cells.
Their affordability, proven safety profile, and widespread insurance coverage ensure continued dominance, even as disease-modifying drugs gain traction.
Combination Drugs Market
Combination therapies—typically pairing a cholinesterase inhibitor with an NMDA receptor antagonist—are increasingly prescribed for moderate to severe Alzheimer’s cases. These regimens offer synergistic benefits by targeting multiple neurological pathways while improving patient adherence through simplified dosing.
United States Alzheimer’s Drugs Market by Drug Type
Galantamine Market
Galantamine is valued for its dual mechanism of action, combining cholinesterase inhibition with nicotinic receptor modulation. It is frequently prescribed as an alternative for patients who cannot tolerate donepezil.
Availability in extended-release formulations enhances patient compliance, sustaining its relevance in neurology and geriatric care settings.
Donepezil Market
Donepezil, widely known by its brand Aricept, is the most prescribed Alzheimer’s drug in the United States. Approved for all disease stages, it is often the first-line therapy following diagnosis.
Generic availability, once-daily dosing, and strong physician familiarity ensure donepezil’s continued leadership in the U.S. Alzheimer’s drugs market.
United States Alzheimer’s Drugs Market by Distribution Channel
Hospital Pharmacies
Hospitals play a central role in Alzheimer’s diagnosis and treatment initiation. Multidisciplinary teams manage complex therapies, monitor side effects, and conduct clinical trials for novel drugs.
Hospital pharmacies are critical distribution hubs for both branded and generic Alzheimer’s medications, particularly for advanced-stage patients.
Online Pharmacies
The online pharmacy segment is expanding rapidly, driven by digital health adoption and caregiver convenience. Auto-refill services, teleconsultations, and home delivery have made online platforms a preferred option for chronic medication management.
Regulatory support for telemedicine has further legitimized this channel, positioning it as a key growth driver through 2033.
State-Level Insights
California Alzheimer’s Drugs Market
California leads the U.S. Alzheimer’s drugs market due to its large elderly population, advanced healthcare infrastructure, and strong biotech ecosystem. Academic research centers and government-supported healthcare programs enhance access to innovative therapies.
New York Alzheimer’s Drugs Market
New York’s dense network of hospitals, neurologists, and research institutions supports early diagnosis and advanced treatment adoption. Medicaid coverage and eldercare initiatives further strengthen market demand.
Florida Alzheimer’s Drugs Market
Florida’s sizable retiree population makes it one of the most significant Alzheimer’s drug markets in the U.S. The presence of memory care facilities and active clinical trial recruitment supports both generic and premium drug utilization.
Market Segmentation Summary
By Drug Class
Cholinesterase Inhibitors
NMDA Receptor Antagonists
Combination Drugs
Others
By Drug Type
Galantamine
Donepezil
Memantine
Rivastigmine
Others
By Distribution Channel
Hospital Pharmacies
Retail Pharmacies
Online Pharmacies
Top States Covered
California, Texas, New York, Florida, Illinois, Pennsylvania, Ohio, Georgia, Washington, New Jersey, Rest of the U.S.
Competitive Landscape and Key Players
The U.S. Alzheimer’s drugs market is highly competitive, with global pharmaceutical leaders investing heavily in R&D and commercialization. Key players include:
AbbVie Inc.
AstraZeneca PLC
Biogen Inc.
Eisai Co. Ltd.
Eli Lilly and Company
Novartis AG
Pfizer Inc.
Each company is analyzed across five viewpoints: overview, key personnel, recent developments, SWOT analysis, and revenue performance.
Final Thoughts
The United States Alzheimer’s Drugs Market is undergoing a historic transition from symptom management to disease modification. While challenges related to affordability and diagnostics persist, strong government support, scientific innovation, and rising awareness are reshaping the treatment landscape.
As research pipelines mature and access improves, Alzheimer’s drugs are set to play a transformative role in improving patient outcomes and reducing the long-term societal burden of neurodegenerative disease. The period from 2025 to 2033 will be decisive in defining the future of Alzheimer’s care in the United States.
About the Creator
Renub Research
Renub Research is a Market Research and Consulting Company. We have more than 15 years of experience especially in international Business-to-Business Researches, Surveys and Consulting. Call Us : +1-478-202-3244






Comments
There are no comments for this story
Be the first to respond and start the conversation.