GCC Liquid Biopsy Market Size and Forecast 2025–2033
Non-Invasive Diagnostics Transforming Cancer Detection and Precision Medicine Across the Gulf
Introduction
The healthcare landscape in the Gulf Cooperation Council (GCC) is undergoing a profound transformation, driven by technological innovation, rising disease prevalence, and strategic government investments. Among the most promising advancements reshaping diagnostic medicine is liquid biopsy—a non-invasive technique that enables early disease detection and real-time monitoring through a simple blood sample.
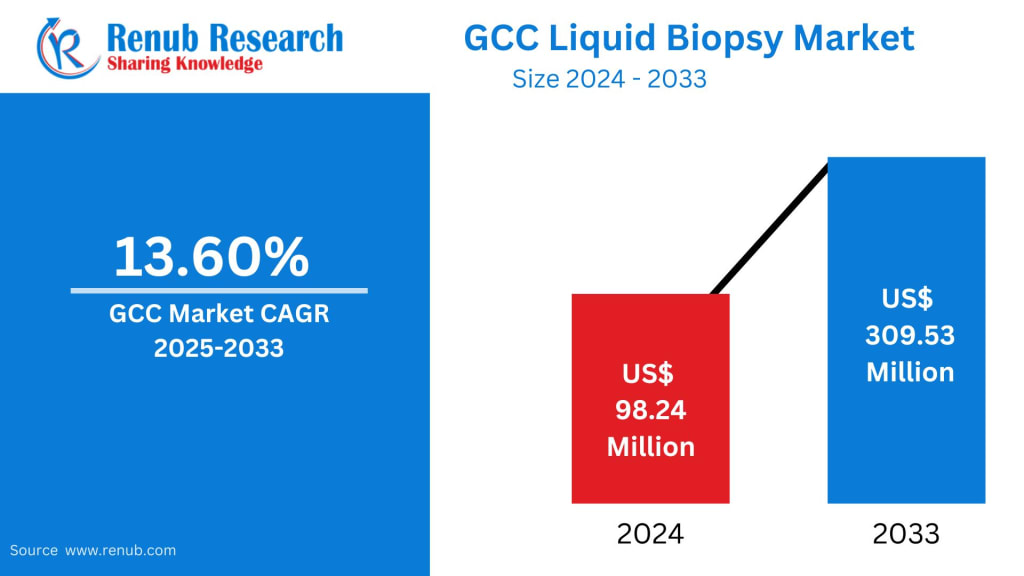
According to Renub Research, the GCC Liquid Biopsy Market was valued at US$ 98.24 million in 2024 and is projected to reach US$ 309.53 million by 2033, growing at an impressive compound annual growth rate (CAGR) of 13.60% from 2025 to 2033. This rapid growth reflects increasing cancer incidence, expanding genomics capabilities, rising healthcare expenditure, and a growing shift toward precision and personalized medicine across GCC countries.
As the region strengthens its healthcare infrastructure and embraces cutting-edge diagnostics, liquid biopsy is emerging as a cornerstone technology in modern oncology and beyond.
What Is Liquid Biopsy and Why It Matters
Liquid biopsy is an advanced diagnostic approach that analyzes disease-related biomarkers found in bodily fluids such as blood, urine, or saliva. Unlike traditional tissue biopsies, which are invasive and often painful, liquid biopsies detect circulating biomarkers including:
Circulating Tumor DNA (ctDNA)
Circulating Tumor Cells (CTCs)
Cell-free DNA (cfDNA)
RNA fragments and extracellular vesicles
These biomarkers provide critical insights into tumor genetics, disease progression, treatment response, and early recurrence. Liquid biopsy enables clinicians to monitor cancer evolution in real time, making it particularly valuable for guiding targeted therapies and personalized treatment strategies.
In the GCC, where improving early diagnosis and reducing invasive procedures are top healthcare priorities, liquid biopsy represents a transformative diagnostic solution with wide clinical potential.
GCC Liquid Biopsy Market Overview
The GCC liquid biopsy market is experiencing accelerated adoption due to a convergence of medical need and technological readiness. Rising cancer prevalence, particularly breast, colorectal, lung, and prostate cancers, has created an urgent demand for early and accurate diagnostics. Liquid biopsy addresses this need by offering faster results, minimal patient discomfort, and repeat testing capabilities.
Additionally, improvements in next-generation sequencing (NGS), digital PCR, and bioinformatics have significantly enhanced the sensitivity and reliability of liquid biopsy assays. These advancements are making liquid biopsy more clinically viable and increasingly accessible across hospitals, diagnostic laboratories, and research institutes in the GCC.
Key Growth Drivers of the GCC Liquid Biopsy Market
1. Rising Cancer Incidence Across the GCC
Cancer incidence is steadily increasing across the Middle East, driven by aging populations, lifestyle changes, and environmental factors. According to international health organizations, cancer cases in the region are expected to rise sharply over the next decade, placing enormous pressure on healthcare systems.
Early detection is critical to improving survival rates, and liquid biopsy offers a powerful solution by identifying cancer-related mutations at early stages. Its ability to detect minimal residual disease and recurrence makes it especially valuable in long-term cancer management. As awareness grows among healthcare professionals and patients, demand for liquid biopsy testing continues to rise across GCC countries.
2. Technological Advancements in Genomics and Molecular Diagnostics
Technological innovation remains one of the strongest drivers of market growth. Advances in NGS, digital PCR, and microfluidics have dramatically improved the accuracy, sensitivity, and scalability of liquid biopsy tests.
These technologies allow clinicians to detect even trace amounts of ctDNA in blood samples, enabling earlier diagnosis and precise monitoring of treatment effectiveness. Additionally, the integration of artificial intelligence (AI) and advanced data analytics is further enhancing diagnostic interpretation and clinical decision-making.
As these technologies become more cost-efficient and widely available, liquid biopsy adoption across the GCC is expected to accelerate.
3. Government Support and Healthcare Investments
Governments across the GCC are actively investing in healthcare modernization, with cancer care and precision medicine identified as strategic priorities. National healthcare visions and long-term development plans emphasize early diagnosis, advanced research, and the adoption of innovative medical technologies.
Public-private partnerships, research grants, and collaborations with global biotech firms are helping expand access to liquid biopsy technologies. These initiatives are strengthening cancer diagnostic infrastructure and encouraging local research and development, positioning liquid biopsy as a key component of the region’s future healthcare ecosystem.
4. Growing Emphasis on Personalized and Precision Medicine
The shift toward personalized medicine is reshaping treatment approaches across the GCC. Liquid biopsy supports this transition by enabling treatment customization based on individual genetic profiles.
By monitoring how tumors evolve and respond to therapy, clinicians can adjust treatment strategies in real time, improving outcomes and reducing unnecessary interventions. This aligns perfectly with the GCC’s goal of delivering high-quality, patient-centric healthcare.
Challenges Facing the GCC Liquid Biopsy Market
High Cost of Advanced Technologies
Despite its advantages, liquid biopsy remains relatively expensive. High costs associated with sequencing equipment, specialized reagents, and skilled personnel limit adoption, particularly in smaller healthcare facilities. Inconsistent insurance reimbursement further restricts patient access, making affordability a key challenge for widespread market penetration.
Regulatory and Standardization Barriers
The absence of harmonized regulatory frameworks across GCC countries poses another challenge. Approval processes vary by country, leading to delays in product commercialization and regional expansion. Clear regulatory guidelines specific to liquid biopsy technologies are essential to support innovation and ensure consistent clinical adoption across the region.
Country-Wise Insights
Saudi Arabia Liquid Biopsy Market
Saudi Arabia represents the largest market in the GCC due to its sizable population and expanding healthcare infrastructure. Under Vision 2030, the country is investing heavily in advanced diagnostics, oncology centers, and biomedical research. Adoption of NGS-based liquid biopsy solutions is increasing, particularly for early cancer detection and treatment monitoring, despite challenges related to cost and awareness.
United Arab Emirates Liquid Biopsy Market
The UAE liquid biopsy market is growing rapidly, supported by high healthcare spending, strong private sector participation, and a focus on medical innovation. Hospitals and research institutions are increasingly integrating liquid biopsy into routine oncology diagnostics, making the UAE a regional leader in precision medicine adoption.
Oman Liquid Biopsy Market
Oman’s liquid biopsy market is expanding steadily as the country enhances its diagnostic capabilities and emphasizes early cancer detection. Growing investment in healthcare infrastructure and increasing physician awareness are expected to drive adoption in the coming years.
GCC Liquid Biopsy Market Segmentation
By Product
Kits & Reagents
Platforms & Instruments
Services
By Application
Cancer Therapeutic Application
Reproductive Health
Other Therapeutic Applications
By Circulating Biomarkers
Circulating Tumor Cells (CTCs)
Circulating Tumor DNA (ctDNA)
Cell-free DNA (cfDNA)
Extracellular Vesicles
Other Biomarkers
By End User
Hospitals
Diagnostic Laboratories
Point-of-Care Testing
Academic Institutes
Others
By Country
Saudi Arabia
United Arab Emirates
Kuwait
Qatar
Oman
Bahrain
Competitive Landscape and Company Analysis
The GCC liquid biopsy market features global biotechnology leaders and diagnostics companies expanding their regional presence. Market players are evaluated across four key dimensions: company overview, key leadership, recent developments, and financial insights.
Key Companies Covered:
F. Hoffmann-La Roche Ltd.
Bio-Rad Laboratories
Thermo Fisher Scientific Inc.
Johnson & Johnson
Guardant Health Inc.
QIAGEN N.V.
Sysmex
These companies are investing in advanced assay development, strategic partnerships, and regional expansion to strengthen their competitive positions.
Final Thoughts
The GCC Liquid Biopsy Market is poised for strong and sustained growth over the next decade. With the market expected to triple in value from US$ 98.24 million in 2024 to US$ 309.53 million by 2033, liquid biopsy is rapidly transitioning from a niche diagnostic tool to a mainstream component of modern healthcare.
Driven by rising cancer incidence, technological breakthroughs, supportive government initiatives, and a growing focus on personalized medicine, liquid biopsy is reshaping disease detection and treatment across the GCC. While challenges related to cost and regulation remain, continued investment, innovation, and policy alignment are expected to unlock the full potential of this transformative technology.
As the GCC advances toward a future of precision healthcare, liquid biopsy will play a vital role in improving patient outcomes, enhancing diagnostic accuracy, and redefining cancer care in the region.
About the Creator
Diya Dey
Market Analyst






Comments
There are no comments for this story
Be the first to respond and start the conversation.