Denmark Pharmaceutical Market Size and Forecast 2025–2033
Innovation-Driven Growth Backed by R&D Strength, Digital Health Data, and Global Pharma Leaders
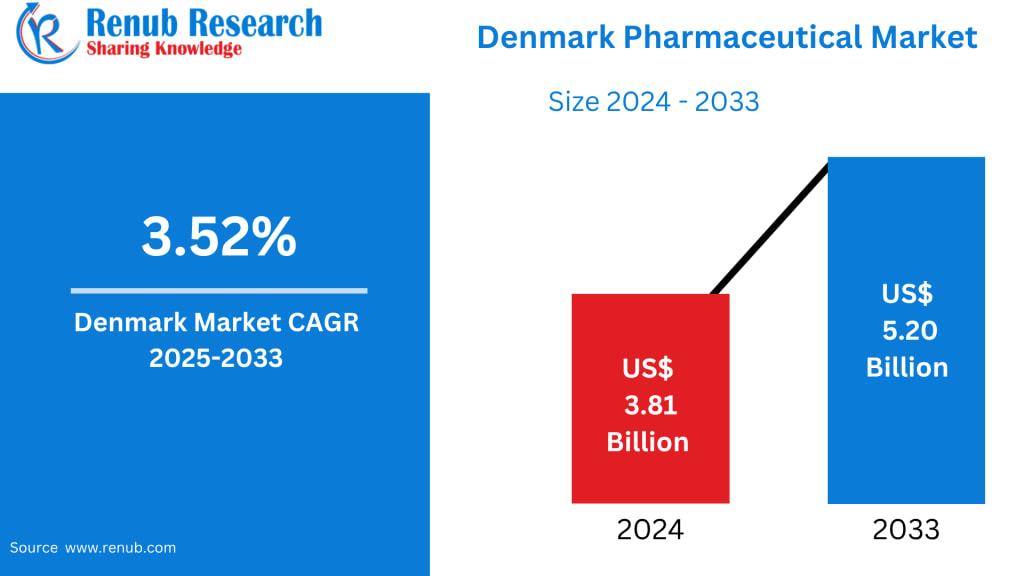
Denmark Pharmaceutical Market Outlook at a Glance
According to Renub Research, the Denmark Pharmaceutical Market is projected to grow from US$ 3.81 billion in 2024 to US$ 5.20 billion by 2033, registering a compound annual growth rate (CAGR) of 3.52% from 2025 to 2033. This steady expansion reflects Denmark’s strong fundamentals—robust research and development spending, a highly digitized healthcare ecosystem, government-backed life sciences policies, and the global footprint of world-class pharmaceutical companies.
Denmark has long been recognized as one of Europe’s most innovation-friendly pharmaceutical hubs. The country combines scientific excellence with regulatory efficiency, making it a preferred destination for clinical trials, biologics manufacturing, and specialty drug development. With rising global demand for chronic disease treatments and biologics, Denmark’s pharmaceutical sector is well-positioned for sustainable long-term growth.
Denmark Pharmaceutical Market Overview
The pharmaceutical sector encompasses the research, development, manufacturing, and commercialization of medicines used to prevent, diagnose, and treat diseases. In Denmark, this industry plays a central role not only in public health but also in the national economy, accounting for a significant share of exports and private-sector R&D investment.
Denmark’s pharmaceutical ecosystem is distinguished by close collaboration between academia, hospitals, biotech startups, and multinational corporations. Prescription medicines, vaccines, biologics, and over-the-counter (OTC) products form the backbone of the market. Strict regulatory oversight ensures drug safety and efficacy, while streamlined approval pathways encourage innovation and speed-to-market.
Global pharmaceutical leaders such as Novo Nordisk A/S and H. Lundbeck A/S anchor the industry, driving exports and reinforcing Denmark’s reputation as a life sciences powerhouse. Combined with a highly skilled workforce and access to the broader EU market, Denmark offers a future-proof environment for pharmaceutical growth.
Key Growth Drivers of the Denmark Pharmaceutical Market
Advanced Health Data Infrastructure
One of Denmark’s most powerful competitive advantages is its advanced and integrated health data infrastructure. The country maintains nationwide electronic health records, disease registries, and biobanks, all linked through a unique personal identification number assigned to every citizen. This enables seamless data integration across hospitals, clinics, and pharmacies.
For pharmaceutical companies, this infrastructure unlocks unparalleled access to real-world evidence. It accelerates clinical trials, improves patient recruitment accuracy, supports post-marketing surveillance, and facilitates personalized medicine development. Researchers can conduct long-term follow-ups with high data integrity, significantly reducing both time and cost in drug development. Strong data protection laws further enhance Denmark’s appeal as a trusted global research partner.
Supportive Government Policies and Life Sciences Strategy
Government support is a cornerstone of Denmark’s pharmaceutical success. The Danish government has implemented a comprehensive Life Sciences Growth Plan, featuring 36 initiatives across clinical research, regulatory efficiency, manufacturing, and innovation ecosystems. These policies aim to strengthen Denmark’s position as one of the world’s leading life sciences nations.
A key highlight is the creation of a “one-stop-shop” regulatory framework that simplifies approval processes for clinical trials and pharmaceutical manufacturing. This reduces administrative burdens and encourages foreign direct investment. Additionally, Denmark’s climate partnership agreements with the pharmaceutical industry promote sustainable production while aligning with EU environmental goals. Together, these initiatives create a stable, innovation-friendly environment for pharma companies.
Strong R&D Investment and Academic Collaboration
Denmark ranks among the top countries globally in R&D expenditure as a percentage of GDP, with life sciences receiving a substantial share. Both public funding and private investment—particularly from major pharmaceutical players—fuel innovation across biotechnology, biologics, and precision medicine.
Universities, hospitals, and industry players collaborate closely, fostering a dynamic research ecosystem. Government incentives such as tax credits, grants, and innovation subsidies further stimulate R&D activities. This environment accelerates the discovery of novel therapies, strengthens Denmark’s clinical trial capabilities, and attracts international research partnerships, reinforcing its global leadership in pharmaceutical innovation.
Challenges Facing the Denmark Pharmaceutical Market
Regulatory Complexity and EU Compliance
Despite its efficiency, Denmark’s pharmaceutical sector must navigate complex regulatory frameworks that combine national regulations with European Union compliance requirements. Meeting both sets of standards can lengthen approval timelines and increase development costs, particularly for startups and small-to-medium enterprises.
Frequent updates to EU pharmaceutical regulations require continuous adaptation, placing additional strain on resources. Balancing regulatory compliance with the need for rapid innovation remains an ongoing challenge for the industry.
Sustainability and the Green Transition
Sustainability is becoming a critical challenge for Denmark’s pharmaceutical manufacturers. The industry faces increasing pressure to reduce carbon emissions, manage water consumption, and minimize waste throughout production processes. Meeting Denmark’s ambitious climate targets and EU environmental regulations requires significant investment in green technologies and supply chain transformation.
While sustainability initiatives enhance long-term resilience and brand value, they also introduce cost and operational complexities. Successfully integrating environmentally responsible practices without compromising efficiency will be essential for maintaining global competitiveness.
Recent Developments in the Denmark Pharmaceutical Industry
July 2022: Bavarian Nordic expanded its production capacity to manufacture up to 10 million doses of the monkeypox vaccine, strengthening Denmark’s role in global vaccine supply.
June 2022: Novo Nordisk A/S partnered with Echosens to promote early detection of non-alcoholic steatohepatitis (NASH) through advanced liver diagnostic technologies.
These developments highlight Denmark’s leadership in vaccines, chronic disease management, and diagnostic innovation.
Denmark Pharmaceutical Market Segmentation Analysis
By Therapeutic Class
Cancer
Infectious Diseases
Cardiovascular Diseases
Diabetes
Respiratory Diseases
Central Nervous System Disorders
Autoimmune Diseases
Others
Diabetes and CNS disorders remain particularly strong segments, driven by global disease prevalence and Denmark’s expertise in specialty therapeutics.
By Drug Type
Branded
Generic
Branded drugs dominate due to strong innovation pipelines, while generics ensure cost-effective access to essential medicines.
By Prescription Type
OTC Drugs
Prescription Drugs
Prescription drugs account for the majority share, supported by advanced diagnostics and physician-led treatment models.
By Distribution Channel
Hospital Pharmacy
Retail Pharmacy
Others
Hospital pharmacies play a vital role in specialty and biologic drug distribution, while retail pharmacies support chronic disease management.
Competitive Landscape and Key Players
The Denmark pharmaceutical market is moderately consolidated, with both multinational leaders and specialized domestic companies. Each company is analyzed across five dimensions: company overview, key persons, recent developments and strategies, SWOT analysis, and sales performance.
Key Players Include:
Novo Nordisk A/S
H. Lundbeck A/S
LEO Pharma A/S
Orifarm Group A/S
ALK-Abelló A/S
Xellia ApS
Takeda Pharma A/S
Sandoz A/S
Ferring Pharmaceuticals A/S
FUJIFILM Diosynth Biotechnologies
Future Outlook: What Lies Ahead for Denmark’s Pharma Sector
Looking ahead to 2033, Denmark’s pharmaceutical market is set to benefit from rising global demand for biologics, personalized medicine, and treatments for chronic and age-related diseases. Advances in biotechnology, AI-driven drug discovery, and real-world data analytics will further enhance productivity and innovation.
Denmark’s strong export orientation, combined with access to EU markets and a trusted regulatory reputation, positions the country as a preferred global pharmaceutical partner. While regulatory complexity and sustainability challenges remain, continued investment, policy support, and technological leadership are expected to offset these pressures.
Final Thoughts
The Denmark Pharmaceutical Market represents a compelling blend of innovation, stability, and global relevance. With market value expected to rise from US$ 3.81 billion in 2024 to US$ 5.20 billion by 2033, the sector demonstrates resilient growth backed by strong R&D capabilities, advanced health data systems, and industry-leading companies.
As Denmark continues to align scientific excellence with sustainable practices and digital transformation, its pharmaceutical industry is well-positioned to remain a cornerstone of European and global healthcare innovation throughout the forecast period of 2025–2033.
About the Creator
Diya Dey
Market Analyst






Comments
There are no comments for this story
Be the first to respond and start the conversation.